- Review
- Open access
- Published:
Rare disease clinical trials in the European Union: navigating regulatory and clinical challenges
Orphanet Journal of Rare Diseases volume 19, Article number: 285 (2024)
Abstract
Background
Clinical development for orphan drugs presents significant difficulties and challenges. There is no unique or standard design, conduct, and outcome assessment methodology and it is sometimes impractical to fit design models of rare disease trials in any practiced and well-known framework. In the European Union (EU) these challenges encompass a broad array of subjects, including trial design, study outcomes, patient recruitment, trial conduct ethics, trial cost, and chances of success. This literature-based review study aims to provide a thorough overview of the critical aspects of rare disease trials in the EU by analyzing the current landscape of rare disease trials, highlighting key challenges, delving into regulatory and research initiatives and innovation in trial designs, and proposing multi-faceted solutions to implement effective rare disease clinical trials in the region.
Discussion
Traditional clinical trial designs, validation, and evaluation methodologies used for nonorphan drugs often prove unsuitable for orphan drugs, given the small patient populations, sometimes fewer than 1000 cases. There is an increasing need for accessible therapies and both regulators as well as industry are trying to develop affordable and effective drugs to address this need. Despite several steps that have been taken, the timely development of drugs remains a challenge. One of the reasons behind the long development timeline is the recruitment, retention, and conduct of rare disease trials. To optimize the development timelines of orphan drugs in the EU, it is important to ensure that the safety and efficacy of the product is not compromised. Industry and regulatory agencies must implement innovative trial designs, devise flexible policies, and incorporate real-world data for assessing clinical outcomes.
Conclusion
Collaboration among academic institutions, pharmaceutical companies (both small and major), patient groups, and health authorities is crucial in overcoming obstacles related to clinical trials and providing assistance and creative ideas. The ultimate objective of granting rare disease patients timely and affordable access to medications with a positive balance between benefits and risks is to be met.
Background
Developing effective treatments for rare diseases is challenging due to their low prevalence in the EU, with only 5 patients per 10,000. Most rare diseases lack adequate treatment options. Conducting clinical trials with small populations is challenging resulting in limited evidence generation. To address this issue, regulatory guidance, such as the “Guideline on clinical trials in small populations” [1] by the European Medicines Agency (EMA) guidance in the EU, covers various aspects of clinical trials, including pharmacological factors, endpoint selection, control group selection, methodological considerations, statistical considerations, and levels of evidence. In 2012, the European Commission (EC) launched a call for proposals, “New methodologies for clinical trials for small population groups,” under the FP7 health innovation framework [2]. This initiative aimed to develop improved statistical methods for assessing the safety and effectiveness of treatments for small population groups, focusing on rare diseases and personalized medicine and increasing evidence generation from the clinical trials to support safety and efficacy outcomes. The objective was to reduce trial design costs and conduct effective clinical trials that produce reliable outcomes for rare disease studies involving small patient populations [3]. Randomized clinical trials have been tried on rare disease trials in the EU, but since the population of patients is small the question of appropriate sample inclusion and impact of outcomes of these trials in the long run cannot be surely concluded [3]. In recent times, innovative clinical trial designs using advanced statistical methods, simulation programs to emulate real-life trial designs and scenarios, and usage of real-world evidence (RWE) to assess and determine clinical endpoints for study design are some of the approaches proposed by regulators.
Significance of the study
This study employs a review of various literature types encompassing review papers, concept papers, points of view, empirical researchers, policy guidelines, and expert reviews to explore the challenges faced by researchers in conducting rare disease trials in the EU as well as patient perspectives, and regulatory hurdles.
It aims to provide key insights by incorporating views and findings from various sources by adopting a fresh perspective considering the evolving landscape of rare disease policies and increasing focus on orphan drug research based on the latest scientific advancements. This study also aims to introduce actionable recommendations beyond theoretical practices and general solutions by incorporating suggestions based on technical advancements in health tracking, disease progression modeling using natural history data, and treatment outcome assessments and provides perspectives for conducting future research in this domain. It focuses on the specific regional nuances of the EU and attempts to identify strategies that will fit into the orphan drug development and regulatory framework of this region.
This study adopts an interdisciplinary approach to promote innovative trial designs led by improved policy decisions, simulation modeling and effective usage of registry and natural history data, novel endpoint and biomarker-based effectiveness assessments, adopting innovative drug development planning, and improving collaboration across the healthcare value chain.
Methodology
Study selection and data collection
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Refer PRISMA Checklist for details. The criteria for the selection of relevant studies and documents and for performing the necessary assessment of relevant literature are provided below.
Search strategy and selection criteria
Search was performed to retrieve relevant studies, policy documents, and online materials from PubMed, SCOPUS, BMJ, JAMA, Nature, Web of Science collection, Taylor and Francis Online, and internet search. Search terms were categorized into three parts:
-
Population: “rare disease OR orphan drugs” AND (Europe OR European Union OR EU OR “multicountry”).
-
Intervention: “clinical trial” OR “clinical research” OR “drug development”.
-
Outcomes: “regulatory challenges” OR “regulatory hurdles” OR “innovative designs” OR “patient recruitment” OR “trial design” OR “methodology” OR “regulatory strategies” OR “real-world data” OR “natural history data”.
The indexing was performed using the following search query:
(“rare disease” OR “orphan drug “) AND (“Europe” OR “European Union” OR “EU” OR “multicountry”) AND ((“clinical trial” OR “clinical research” OR “drug development”) AND (((“regulatory challenge” OR “regulatory hurdle”) OR “regulatory strategy” OR (“innovative design” OR “trial design” OR “methodology”) OR (“patient recruitment” OR “patient engagement”) OR (“real world data” OR “real world evidence”) OR “natural history data”).
The data related to the clinical trial landscape were extracted from the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization and the EU-related data were filtered based on the EU Clinical Trial registry.
We tailored data extraction to fit the study’s qualitative and narrative nature, systematically retrieving key meta-data like document details, publication dates, objectives, designs, policies, shortcomings, opportunities, and findings. Two independent reviewers ensured the accuracy of data by reviewing various aspects covered in the documents.
Inclusion and exclusion criteria
The content considered for inclusion comprised studies that report on the regulatory and clinical challenges of conducting rare disease clinical trials in the EU and focused on regulatory and innovative strategies to address the same, studies including commentaries, reviews, and editorials that provide recommendations or solutions to overcome the challenges of rare disease clinical trials in the EU, studies that are published in English or have an English abstract available and studies that are published from 2005 onwards, to reflect the evolving regulatory and clinical landscape of rare disease research and trials in the EU. Also, articles and web publications in peer-reviewed journals or websites and from reputable organizations were considered for study.
Studies that do not focus on rare disease clinical trials, or only mention them as a secondary or minor aspect, studies that did not address the EU context, or only mention it as a secondary or minor aspect, Studies that provided a generic overview of these aspects and have methodological limitations and studies not directly addressing the specifics of the challenges and not providing any insight into innovative trials and clinical evaluation or planning were excluded from the study.
23 journal articles and 30 web publications (position papers, editorials, frameworks, guidelines, and details of stakeholder organizations in the EU rare disease landscape) were considered to conduct this study.
Discussion
The research focus and findings of the 23 journal articles are provided in Table 1. Please refer below:
The studies of rare disease trials have proposed key conceptual frameworks and have also provided empirical insights into the design, conduct, evaluation, and outcome of these trials. A baseline synthesisation of key findings from these studies provides a core observation that the design and conduct of clinical trials can be assumed to be centered around three themes: Disease characteristics, Trial Design and Methodology, and Clinical Outcome Assessment. The core concept of clinical research plans begins with understanding specific rare diseases, their heterogeneity, and natural history, which inform Trial Design and Methodology, including study objectives, endpoints, and ethical considerations. Clinical outcomes and impacts, such as efficacy, safety, patient-reported outcomes, and cost-effectiveness, are influenced by the chosen methodology and may vary across different ethnic groups and even within patients at various stages of the disease or encountering specific molecular subsets of the disease. Relationships between these themes highlight the iterative nature of clinical research and especially in the case of rare diseases these iterations may not be sequential but in most cases need to be concurrent and flexible to ensure maximum and diverse patient coverage. Incorporating the themes in a common regulatory and funding framework across the EU becomes challenging due to huge ethnic, geographical, financial, infrastructural, and cultural diversity. Stakeholder perspectives, comparative analysis, ethical considerations, and future directions add depth to ongoing drug development initiatives. This approach warrants the inclusion of diverse viewpoints, ethical awareness, ongoing refinement of research practices, and continuous innovation in the design and conduct of trials to address the unique challenges and opportunities in rare disease clinical trials within the EU.
Landscape of orphan drug clinical trials in the EU:
Clinical trials conducted based on therapeutic areas in the EU
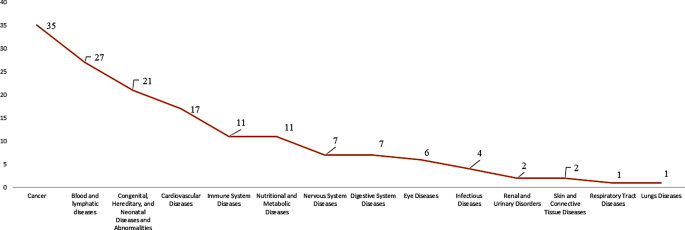
Between 2007 and 2022, 152 clinical trials were conducted on orphan drugs in the EU, as recorded in the EU Clinical Trial Registry. Refer to Fig. 1. 23% of the trials were on cancer-related therapies, followed by blood disorders with an 18% share of the trials and congenital abnormalities with a 14% share of the trials. The rest of the trials were related to other therapeutic areas, such as cardiovascular diseases, immune system diseases, and nervous disorders.
Orphan drug clinical trials based on therapeutic area (2007–2022) (EU Clinical Trial Registry). Source International Clinical Trials Registry Platform (ICTRP) [9]
Yearly spread of clinical trials of orphan drugs in the EU
Between 2007 and 2022, clinical trials on orphan drugs have seen both an increase and a decrease on a year-on-year basis, with a decrease observed during the intermittent period mainly in 2015 and 2016. Refer to Fig. 2. After that, the trend has been upward again, with 22 trials being conducted in 2021, which is the highest in the period under observation. The number again decreased to 12 in 2022. There is an average increase of 51% in the clinical trial volume from 2007 to 2022.
Yearly orphan drug clinical trials in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [9]
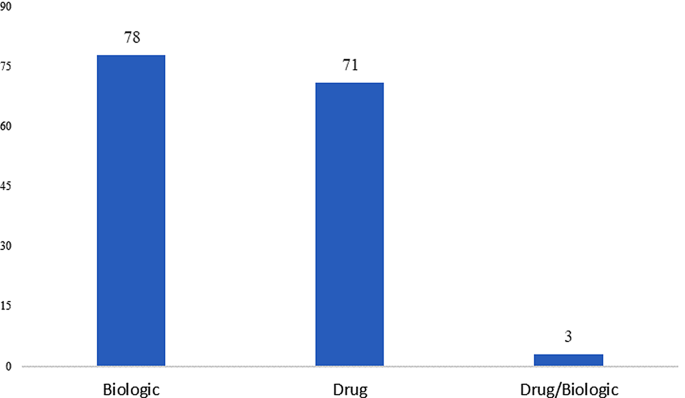
Clinical trial volume of orphan drugs based on product types in the EU
Between 2007 and 2022, biological products underwent 78 trials compared to 71 trials for synthetic drugs. Refer to Fig. 3. This might be an indication that investments made by the EU in the field of biotechnology have created a favorable environment for sponsors for biologic development. The EU market provided potential commercial opportunities due to less competition in the field of biologics. Biologics may also be favored due to their targeted mechanism of action, high specificity and efficacy, and ability to modulate complex biological processes underlying the specific disease being treated. These factors make biologics more suitable therapies than synthetic drugs for diseases belonging to certain therapeutic areas.
Orphan drug trial volume based on product type in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [9]
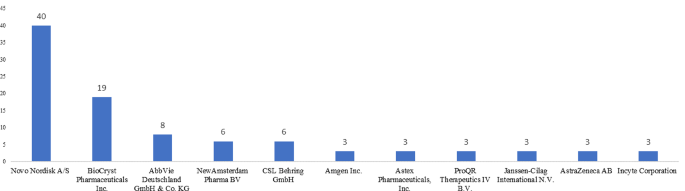
Top 10 companies conducting orphan drug clinical trials in the EU
Between 2007 and 2022, Novo Nordisk conducted the maximum number of clinical trials focusing on congenital, hereditary, and neonatal diseases and abnormalities, nutritional and metabolic diseases, digestive system diseases, cardiovascular diseases, and blood and nervous disorders. It was followed by BioCryst Pharmaceuticals Inc., which conducted 19 trials focusing on blood disorders and immune system diseases. The top 10 companies contributed to 64% of all orphan drug clinical trials in the EU between 2007 and 2022. Refer to Fig. 4.
Top 10 companies in terms of orphan drug trials in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [9]
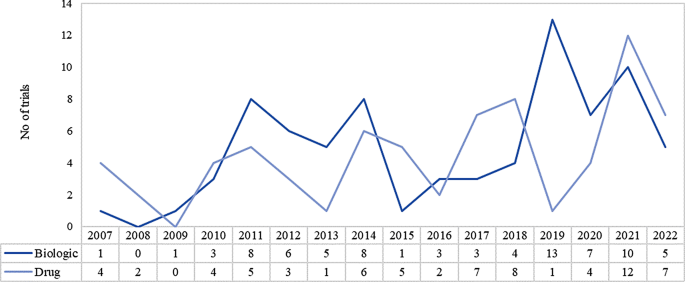
Clinical trials on biologics have seen an increase between 2019 and 2022 primarily due to an increased focus on biologics development for rare disease therapies. This is also backed by the growing knowledge of genetic markers of specific diseases that help in the development of targeted therapies for patients. It was observed that 45% of all biologic therapeutic trials between 2007 and 2022, were carried out between 2019 and 2022. Refer to Fig. 5.
Yearly trend of clinical trials of orphan drugs based on product types in EU (2007–2022). Source International Clinical Trials Registry Platform (ICTRP) [9]
Inferences from landscape data
Companies have been showing an increased interest in orphan drug development in the EU, primarily supported by the EMA’s orphan drug development incentives such as ten years of market exclusivity for orphan-designated products, centralized and accelerated review of marketing authorization applications for orphan products, conditional marketing authorization of certain drug types, compassionate access under exceptional circumstances for patients with high morbidity, application and regulatory fee waivers and EC research frameworks and grants for orphan drug innovation and rare disease natural history studies. Most of the major pharmaceutical companies have developed and conducted trials on a considerable number of orphan drugs across different therapeutic areas. This, supported by collaborative programs by European Reference Networks (ERNs), the European Joint Program on Rare Diseases (EJP-RD) [10, 11], and the International Rare Diseases Research Consortium (IRDiRC) [12] has played a major role in creating a translational research environment for orphan drug development and innovative trial design. Many small and medium enterprises (SMEs) working on rare disease innovation in the biotechnology and pharmaceutical domain are supported by the European Confederation of Pharmaceutical Entrepreneurs (EUCOPE) in terms of financial incentives, development support, and scientific advisory. EUCOPE also acts as a bridge for advancing translational research. Qualified SMEs receive additional incentives from the EMA like a 100% fee reduction for administrative and procedural assistance, pre-authorization inspection, initial marketing authorization application and post-authorization applications, and annual fee, specified in Council Regulation (EC) No 297/9554, in the first year from granting of a marketing authorization. The development of unified patient databases through the European Rare Disease Registry Infrastructure (ERDRI) and Patient-Reported Outcome and Quality of Life Instruments Database (PROQOLID) [13] database by Mapi Research Trust has provided valuable information on Real World Data from patient treatment outcomes, valuable biomarkers, diagnostic reports, and observer as well patient experience feedback [14]. Although there have been definitive steps taken at the EC level, regulatory agency level, and by research consortiums and patient organizations such as the European Organization for Rare Diseases (EURORDIS) [15], the pace of innovation and marketing approval of drugs is not as expected, as is evident from the overall analysis presented in the landscape section Apart from the practical challenges that are discussed in a later section, ineffective utilization of regulatory incentives and the requirement of high capital investment have been factors behind a slower pace of therapeutic development. Along with the key requirements of addressing clinical trial issues, it is equally important to implement an alternative approach to drug development planning to increase the participation of players in orphan drug clinical research and development.
Key aspects of rare disease trial designs
Practical challenges in conducting orphan drug clinical trials in the European Union
Clinical trials, being one of the most significant stages in the drug development lifecycle face various hurdles, especially in a multi-country setup like the EU. It includes finding eligible patients and overcoming awareness issues. International trials introduce further complexities like consensus on diagnosis and cultural considerations [4] Disease heterogeneity poses further challenges making progression tracking difficult, and varied manifestations across different patient groups create challenges in diagnosis, treatment, and understanding effectiveness [5]. Genetic factors, variability in disease frequency among patient groups [3], lack of knowledge about disease progression [7], and co-existing illnesses complicate the determination of inclusion/exclusion criteria and the tenure of trials [8, 16]. In many cases, RCTs fail to deliver the desired trial outcomes in normal settings. Limited knowledge about appropriate endpoints complicates protocol design, hindering regulatory approval. Small patient populations and data variability exacerbate challenges in demonstrating drug effectiveness and safety, compounded by the absence of standardized clinical trial templates for most rare [6, 17]. Balancing risks and benefits, respecting autonomy, and ensuring equal access are crucial issues. The limited treatment options make patients more likely to accept risks, raising concerns about informed consent which becomes a critical ethical issue [17]. Evolving regulations with stricter requirements and limited expert resources can delay research [18]. Regulatory processes and differing policies across countries add complexity to the design and conduct of rare disease trials in the EU [19]. Regulators must rely on literature review information and empirical data to a large extent when making decisions. Data extrapolation is followed for diseases with existing data, while for diseases with little prior data, several iterations need to be performed across different patient population samples resulting in cost overruns or high expenditure for the sponsors. A considerable patient population of rare disease patients is children, posing challenges in recruitment, retention, and management due to factors like developmental, emotional, and family dynamics. The varying clinical research approaches for pediatric and adult use intensify complexities in recruitment criteria, consent, and regulatory acceptance of study outcomes, amplifying oversight of ethical aspects in conducting trials [20]. In terms of costs, rare disease clinical trials incur high costs due to challenges such as the geographical spread of patients, complex trial protocols, and expensive manufacturing overheads to meet regulatory standards. Specialized trial designs may be necessary to recruit the required number of patients, further escalating expenses. Additionally, data collection and analysis using sophisticated statistical models, drive up the overall cost of drug development. Hence, this factor becomes one of the key components in drug pricing, treatment availability, and reimbursement as well. Countries like Germany, with a strong economy and financial budget, have the privilege to establish initial prices of innovative therapies making it one of the initial markets for product launch. This results in a price that may be attractive to the sponsor company but may pose a significant financial burden on countries with less financial resources and undeveloped reimbursement schemes. The downside of high pricing needs to be considered as well. Any kind of failure in price negotiations for high-cost therapies with the national Governments results in the withdrawal of the therapeutic product from the entire EU market due to a lack of reference and attractive pricing for the company. An example of this was the withdrawal of Zynteglo, for the treatment of severe Beta thalassemia and of Skysona, for the treatment of Cerebral adrenoleukodystrophy by Bluebird Bio. Pricing remains a critical issue in rare disease drug development and treatment availability [21]. One approach to mitigate the cost issue, is to implement specialized National Action Plans for rare diseases as per recommendations of the European Council. From an economic point of view, it seems difficult to implement uniform research plans, funding mechanisms, and reimbursement schemes across all member states. To address this, the European Committee of Experts on Rare Diseases (EUCERD) came out with EUROPLAN indicators to monitor the progress of plans in individual member states. These indicators can help track the progress of the National Action Plans. Stronger economies can provide financial and administrative expertise and financial grants through the E-Rare (ERA-NET) research program on rare diseases. An increased public-private partnership should be encouraged to enhance the expertise in conducting clinical research through a multi-stakeholder collaborative approach. This will provide an initial booster to the weaker economies to streamline their action plans and frame appropriate funding and reimbursement mechanisms for rare disease drug development and treatment.
Technical aspects of clinical trial design for rare diseases
RCTs have been the globally accepted and most reliable clinical trial design among clinical researchers, investigators, and regulatory agencies for demonstrating the effectiveness of a drug. However, conducting multiple RCTs can be time-consuming, and expensive, and may not fully reflect real-world clinical settings. As a result, there is a growing interest in finding innovative approaches to enhance the efficiency of clinical research [22]. RCTs are designed with standardized and comprehensive outcome measurements to determine the safety of the drug under trial. RCTs generate substantial evidence to determine the effectiveness of the drug by incorporating bias-reduction techniques to reduce errors in observations [22].
Randomization helps to differentiate between treatment outcomes as well as the variability of outcomes within a group. However, despite the multiple benefits and robustness of the design of RCTs, the validity of these cannot be always confirmed in orphan drug scenarios due to the small patient population. This is due to sampling techniques being unable to provide sufficient observation points. The design of orphan drug trials requires multicentre coordination across different locations in the world [8]. This requires the design and analysis of innovative trials through appropriate randomization procedures for a smaller population of patients. As the patient population varies across diseases as well as across demography or geography, there are always differing amounts of bias. Hence, it is imperative that no unique procedure or randomization technique is applicable, and it should be supported by proper design methodology, statistical tests, and analytical methods [3].
Rare disease clinical trial design considerations
Randomized trials are highly dependent on patient registry information to identify specific representative populations. Periodic reference to the updated registries can help sponsors improve study design and determine study cohorts to conduct case-control and observational studies. This helps in bias reduction but there are also certain challenges in terms of rare disease trial design. Existing registries may not have adequate information and validation of the correctness becomes a challenge due to highly fragmented content, lack of expertise, and data sharing or privacy controls. From a disease perspective, the progression of rare diseases is highly heterogeneous and varies between demographics and geographical dispersions. Genetic markers within the same geographical area can vary between communities with few communities highly susceptible to the disease due to cultural or lifestyle practices. In this scenario, it is difficult to determine the proper natural history and clinical endpoint based on diagnostic and treatment outcomes [3].
Analytical and statistical considerations for clinical trial designs
While statistical sampling and analytical methodologies are proven to be highly effective in identifying patient subsets and trial effectiveness outcomes for RCTs for nonorphan drug trials, the same methodologies may not prove effective in the case of rare disease trial designs. It is important to identify efficient design and analysis techniques. While it is recommended to adopt well-defined, widely used, and regulatory-approved techniques, evaluations need to be performed and continuous monitoring is required to establish method validity and adaptability to various trial designs for rare diseases [3]. In studies of rare diseases, small sample sizes can severely restrict design options, hinder the usage of standard statistical models as mandated by regulators, and make replication of study models difficult. To assess the efficacy and safety of potential treatments, it is imperative to explore novel and innovative statistical designs [23]. It is also important to identify an appropriate observation population and study cohort to ensure that bias is properly addressed by using computer simulation models and advanced statistical techniques. While computer simulation methodologies can address the population issue to a considerable extent by identifying patient cohorts for randomized studies; it is also important to systematically study the interactions between treatment and disease phenotypes to prepare targeted trials to understand drug-patient interactions under personalized settings and individual requirements. Statistical algorithmic models can prove helpful in this regard. Digital endpoints generated from sensors installed in wearables can provide valuable real-time data that can be fed to computer simulation models to identify appropriate biomarkers and generate clinically relevant endpoints or surrogate endpoints [24]. However, there is a challenge, as identifying relevant and meaningful data from multiple observations in the form of digital data is time-consuming and requires multiple iterations.
Recommendations for improving the clinical trial design of rare diseases
Effective usage of natural history and disease registry data for trial design
The strategic utilization of natural history and disease registry data plays a pivotal role in the design of clinical trials for rare diseases. This data serves as a roadmap, providing valuable insights into the complex and unique progression trajectories of these diseases, which is an essential component in assessing the impact of new therapeutic interventions [25]. It is important to harness the crucial information around these studies to derive quantifiable biomarkers to effectively chart the therapeutic roadmap, design the trial along with devising a robust drug development plan to augment the possibilities of regulatory approval supported by appropriate safety and efficacy data. Studies have been conducted on Duchenne Muscular Dystrophy based on natural history data for identification of relevant biomarkers based on progression and severity and these data provided valuable data for regulatory assessment [26, 27]. It is to be noted that the landscape of natural history studies on rare diseases faces multiple challenges, including a limited pool of participants, issues with data quality, and the existence of data silos [28]. However, innovative solutions to design simulation models utilizing machine learning, are emerging to address these obstacles, fostering an environment of collaboration among stakeholders and promoting shared learning to enhance knowledge and expedite orphan drug discovery. Properly designed and maintained non-proprietary patient and disease registries based on real-world data, clinician and patient-reported outcomes and natural history study databases are cornerstones in this endeavor, offering a wealth of information about rare diseases [23]. These resources enable the design of robust clinical trials equipped with outcome measures that are both relevant and clinically meaningful. By harnessing this data, researchers can significantly enhance the design of clinical trials for rare diseases. This will lead to the development of more effective therapies in the EU, enable informed regulatory approvals, and pave the way for improved patient outcomes, marking a significant stride in developing and implementing a robust patient-centered approach toward orphan drug development.
Adoption of innovative clinical trial design
In clinical trial designs for orphan drugs, the process of obtaining approvals should take into account the outcome variations and underlying variability in disease manifestations. Due to the lack of a sufficient population, Phase 3 studies for orphan drug approvals tend to include a smaller number of patients, do not have placebo controls in many cases, and employ nonrandomized and unblinded trial designs, such as a single-arm design and surrogate endpoints, for assessing efficacy [3]. This requires proper planning, collaboration, and timeliness. As in most cases, rare disease trials involve multiple sites, and consensus is required between researchers and regulatory agencies in terms of the definition and classification of the disease under trial, identification of appropriate biomarkers and endpoints, and assessment of outcomes on commonly accepted standards. Under these circumstances, innovative trial designs such as basket trials and umbrella trials can help to address these issues. Basket trials enable researchers to investigate certain disease types with specific genetic biomarkers under a common trial design and protocol. Using this approach, researchers test the efficacy of targeted therapies by grouping patients based on molecular characteristics of the disease and provide valuable insights into the potential benefits of a treatment in rare diseases. These trials use a common targeted intervention. On the other hand, umbrella trials involve enrolling patients with the same disease but with different molecular or genetic subtypes. This design allows researchers to test multiple targeted therapies simultaneously within the same disease population, including rare diseases. Umbrella trials help identify which subgroups of patients may benefit from specific treatments, leading to more personalized and effective interventions. These trials use multiple targeted interventions. Both of these trials can also be designed using control groups through randomization, thus ensuring bias reduction and effective assessment of clinical outcomes. These trials have certain advantages, such as sharing the same control group to improve efficiency, reducing the likelihood of patients receiving a placebo, allowing comparisons between active substances and pooling of data from active treatments, and sharing of resources, thus leading to reduced trial costs and more efficient use of trial logistics [16]. In a rare disease setting, these trials may face some challenges around sponsor coordination in terms of multiple treatment trials, complex study design, competing and conflicting interests among stakeholders, operational challenges when international centers and multiple sites are involved, and implementing and following a common protocol. In this scenario, the ERICA, ERNs, patient advocacy groups, and centers of excellence can play an active role in identifying suitable patient populations and can act as co-ordinators or collaborators for designing, funding, and assisting in conducting trials through disease expertise, data sharing, execution management and assessing the outcomes of trials in multicenter settings [16].
Adaptive trial design for rare disease trials
Adaptive trial designs can offer flexibility and increase the efficacy of rare disease trials. Adaptive design trials encompass modified randomization procedures, the addition or discontinuation of treatment arms or doses, sample size adjustments based on interim results, adaptive patient population enrichment, and the incorporation of prespecified rules for efficacy. These designs in exploratory settings allow for the evaluation of various doses, regimens, and populations, focusing on the most favorable observations that will ensure promising results. They increase flexibility and acceptability and maximize the trial’s potential based on gathered data. Prespecified modifications maintain validity and integrity while adjusting elements of the study design [29]. The statistical approach of these trial designs enables modifications of study elements for minimizing errors, careful planning, and ensuring trial ethics and integrity [8]. While designing these trials, it is important to address and mitigate challenges around operational logistics, feasibility, and access to technical expertise. These are crucial considerations in designing clinical trials and special attention should be given to rare disease trials. Adequate study design expertise is necessary to ensure appropriate planning, for which experienced clinical researchers, statisticians, and healthcare professionals who are well-versed in rare diseases and orphan drug trial design, execution, and outcome assessment should be identified. Additionally, maintaining data and trial integrity becomes essential for post-interim analyses. This requires data storage and analytics planning. Addressing concerns related to bias in estimated treatment effects further strengthens the integrity of the trial, and outcomes and observations become more acceptable to regulators [8].
Clinical trials through inferentially seamless adaptive designs
In the adaptive seamless design, trials are merged, and analyses are seamlessly integrated by including data from patients enrolled both before and after the adaptation in the final analysis [30]. Adaptive seamless designs, particularly in the context of rare diseases, offer an appealing approach when traditional group sequential designs for assessing efficacy or futility may not be feasible due to limited sample sizes [31]. These designs integrate a Phase 2 study, which focuses on treatment selection, with a Phase 3 study for confirmatory testing. This integration allows for treatment selection and the re-evaluation of sample size at a predefined interim analysis [3]. The use of adaptive seamless designs in rare disease clinical trials offers several benefits. It maximizes patient data utilization, leading to stronger conclusions, while reducing the number of patients and saving time and costs in Phase 3. It improves target dose and participant selection, explores covariates between Phase 2 endpoints and Phase 3 outcomes, and provides valuable information on treatment effects and safety by following patients from terminated treatment groups. Additionally, it allows for treatment modifications, enhancing the chances of patients receiving safe and effective treatments [32]. Challenges arise when working with these designs, including the time required for their design and the need for appropriate analyses to account for potential bias in treatment effect estimates due to data combinations at different study phases [16].
Decentralized clinical trial design for rare diseases
Decentralized methods involve conducting assessments in alternative locations such as participants’ homes, local clinics, or digital platforms on mobile devices or computers and not at centralized medical facilities. Decentralized clinical trial (DCT) approaches that incorporate physical and virtual consultations, along with online access to medicines or providing drugs through local clinicians or pharmacists, can bring substantial benefits by reducing the burden on patients and their families [33]. DCTs do not completely remove the physical interactions between clinicians and patients. It leverages digital health technologies (DHTs) such as medical devices and wearables. for electronic collection and usage of reliable diagnostic and clinical data, clinical outcome assessments (COAs), Patient and Observer Reported Outcomes, and clinical health records. These enable clinicians to determine exploratory patient-relevant endpoints, enable targeted patient recruitment, and help in collecting and updating data registries for secondary usage in future trials as reference points. This integrated approach will help clinical trial design be more focused on the outcomes. DCTs have their setup challenges in terms of technology adoption and usage, data privacy and technology literacy concerns, and site readiness with appropriate infrastructure, trained personnel, and logistical support. Keeping in mind the needs of patients, industry and clinical stakeholders need to upgrade technical aspects and knowledge of data collection and analysis [34]. Addressing data privacy concerns while sharing data across multiple trial sites as per domestic and international regulations will ensure confidentiality and appropriate usage of data. If the challenges are properly addressed, DCTs can play a crucial role in terms of increased adoption in conducting rare disease clinical trials and acceptance across regulators during decision-making.
Usage of external controls in designing effective rare disease trials
As rare disease clinical trials are subjected to small patient populations, nonrandomization has been adopted in multiple scenarios. Nonrandomized clinical trials that compare against external controls have proven effective in an expanding range of cases, especially in the context of rare diseases. These designs are particularly valuable when randomization is impractical or ethically challenging, or when the available pool of patients with a specific condition is limited [22]. Clinical trial researchers need to understand disease progression and interpret measurements accordingly, but precise methods often don’t translate well to real-world practice. Control groups in these studies are generally being replaced with historical controls based on natural history data. Natural history studies provide a context for a “dry run” of clinical trials, facilitate biological endpoint selection, help in designing an informed clinical trial program with appropriate inclusion/exclusion criteria, help in selecting proper biomarkers for treatment delivery [28], and help to understand long-term trial issues. Errors in this stage are less costly and can inform improvements in the actual trial. Clinical assessments are performed accordingly under small patient population settings providing contextual evidence for regulatory assessment. Incorporating external controls in clinical trials requires meticulous analysis and adept adjustment. Controls are chosen from data obtained from registries, medical records, and scientific literature. Data are also obtained from data from expanded access programs, which provide access to investigational treatments for patients with serious or life-threatening conditions [22]. The process of utilizing external controls entails rigorous statistical methods that help minimize potential biases that can influence results and ensure the validity and reliability of the trial findings.
Protocol flexibility for conducting rare disease clinical trials
In many situations, regulators have provided flexibility to sponsors and trial organizers for rare disease clinical trials. Placebo control can be omitted, trial design can be unblinded and nonrandomized, surrogate endpoints can be used for efficacy assessment and trials can be single-arm [3]. Another possible approach is the substitution of clinical endpoints with biomarkers, ideally in the form of a panel of biomarkers representing various aspects of the disease [28] In the case of rare diseases with insufficient biomarkers, the totality of trends in clinical efficacy and safety data can be assessed by utilizing the entire body of available evidence followed by response simulations from clinical trials and pharmacological modeling of data based on reliable biomarkers [25]. These enable adjusting sample sizes, treatment arms, or endpoints, to ensure the most efficient use of resources and maximize the chances of obtaining meaningful results. The preference or requirement for evidence about safety from RCTs must be still present, as these are based on feasibility considerations, such as the practicality of measuring and monitoring safety levels. However, small population trials may not provide sufficient information about safety or efficacy in the long term. To address the unmet medical needs of patients and prioritize public health, it may be possible to consider granting marketing authorizations with less comprehensive data than typically needed [8]. Hence, ongoing monitoring and data collection of a new medicine is being proposed for evaluating efficacy endpoints. It involves monitoring safety, minimizing risks, conducting additional studies for more knowledge, and evaluating risk-minimization strategies. This helps gather valuable insights to enhance understanding and management of the medicine’s benefits and risks [31]. Regulators can provide the flexibility to sponsors to devise a plan for data monitoring and continuous feedback rather than solely relying on trial outcomes. Additionally, protocol flexibility facilitates the inclusion of novel trial methodologies, such as basket or umbrella trials, which can evaluate multiple treatments or subgroups within the rare disease population simultaneously [35]. By embracing protocol flexibility, researchers can address rare disease trial-specific challenges, apply statistical models for design and outcome analysis that are tailored to adaptive trial settings, and improve the chances of successful outcomes and the development of effective therapies for patients with rare diseases. Another aspect regarding adopting flexible protocol is to undertake a multi-faceted approach to train assessors in data evaluation for rare diseases, to enable them to approve applications based on limited data. This includes a deeper understanding of disease heterogeneity, an understanding of the uniqueness of disease manifestation through direct patient interaction, the adoption of machine learning and iterative approaches in data assessment, and ongoing skill enhancement programs. Specific training programs in line with the National Institute of Health (NIH) funded R25 Rare Disease Clinical Research Training Program can impart specialized skills to assessors in rare disease clinical data assessment. To promote innovation in clinical trial design, the Scientific Advice Working Party (SAWP) under the Committee for Medicinal Products for Human Use (CHMP) of the EMA administers a process to evaluate and qualify innovative methodologies for drug development. This involves providing scientific advice and opinions on various methodologies, such as the multiple comparison procedure modeling, which is recognized as an effective statistical approach for model-based design and analysis [8].
Data extrapolation for efficacy assessment and re-usage of existing treatment
Data extrapolation can be adopted by researchers by leveraging available data from various sources. This can include using historical control data, real-world evidence, registries, electronic health records, clinical literature, and scientific papers or data from similar diseases or patient populations and applying extrapolation techniques to infer the treatment’s effectiveness. By extrapolating data, researchers can make informed judgments about the efficacy of the treatment in the context of the rare disease [36]. This has been utilized to extend existing treatments in adults to the pediatric population based on scientific evidence and assessing their efficacy. This strategy was adopted as part of a need to address the practical and ethical challenges associated with conducting clinical trials in pediatric patient populations and was discussed in an EMA concept paper. If there are scientifically robust data available for an orphan indication, it might be possible to extend these data to offer substantiating evidence for the reasonable certainty of effectiveness, likely advantage, and safety for another orphan indication or subset of a population. The determination of the amount and reliability of data to be utilized for extrapolation, along with the timing of the extrapolation (whether in early or late phase trials), must be carefully assessed on an individual basis for each case [16]. Data can be obtained from clinical trials, real-world evidence, and non-clinical studies. Early identification of relevant data, in collaboration with regulatory authorities, is crucial and can be aligned with a pediatric investigation plan development during initiating studies in adults [23]. As there is limited experience and educational gap in this area thus far, it is important to assess all the nuances and plan the methodologies and validation models by applying rigorous statistical and scientific methods to ensure the correctness and reliability of the extrapolated results. To address knowledge gaps and skills, it is crucial to implement a skill development initiative and continuous assessment for biostatisticians, clinicians, and data analysts.
Engagement of patients during study design
It is important to engage the currently known patient population through patient advocacy groups, outreach programs, and advertisements while designing clinical trials. Engaging patients at an early stage and continuous feedback and information sharing can help understand the concerns of the relevant group and can streamline various aspects of trial design, such as safety considerations, benefit-risk assessment, and selection of endpoints [16]. It is recommended to consult with patients who have experience with clinical trials, and early engagement is preferable. Study designs that are based on patient preferences can serve as crucial and valuable reference points in the overall drug development process and regulatory decision-making. Patient motivation also plays a crucial role, as any new treatment that can prove to be effective will have increased interest among target groups and continuous engagement gives a sense of responsibility and belonging to the overall process [5]. Patient participation and retention are very crucial in conducting successful orphan drug trials, especially in countries with limited patient populations. Collaboration across international centers, engagement with patient advocacy groups, involvement of specialist centers, and proper patient education by research staff is essential for successful participation [8].
Use of Real-World Data in rare disease trial design
Clinical trials face numerous challenges that hinder the timely delivery of promising treatments to patients. However, the use of real-world data (RWD) is emerging as a tool to improve the pace of therapy development. RWD, collected during routine patient care, patient-reported outcomes, handwritten notes, medical records, charts, electronic health records, registry data, and observer reports is gaining support from regulators and can be applied in three key use cases: synthetic control arms (SCAs) to replace or augment standard control arms in trials, precision registries for adaptive trial design, and clinical trial site feasibility to improve patient enrollment. SCAs based on a patient’s standard of care (SOC), in particular, offer an alternative to traditional control arms, which may be unethical in certain cases and help in the identification of endpoints that may vary based on the patient’s disease condition [37]. RWD also enables researchers to optimize study design, assess site feasibility based on patient demographic information of the site, collect more comprehensive and diverse data, and overcome challenges associated with data quality through the application of statistical methods and machine learning algorithms [38]. The integration of RWD in clinical trials holds promise for advancing the development of therapies by quantifying benefits or risks, thereby increasing the success probability and improving health outcomes for patients [23]. The key challenges lie in ensuring data quality, reliability, and interoperability. As many RWDs are extracted through electronic means there are chances of missing data points, and the quality of data may become compromised when merging with multiple other sources for analysis purposes. It is important to classify the data based on type and source and apply appropriate extraction and transformation procedures before they are analyzed for decision-making. To improve interoperability and to ensure data privacy, tokenizing clinical data will be helpful enabling intersystem operability, reliability of information, and availability and accessibility as and when needed [39]. RWD leads to the generation of appropriate RWEs that enable hybrid study methodologies combining the strengths of RCT studies with RWE such as RWE-RE (Real World Evidence-Randomized Enrichment design), mitigating limitations and potentially enhancing or replacing RCTs in many cases, thus providing a patient-centric approach towards statistical data analysis and supporting regulatory decision making [23].
Some steps taken by the International Council on Harmonization (ICH) are to update the E8 and E6 guidelines to include more flexible study designs and diverse data sources. This includes discussions on pragmatic study designs and guidance on using RWD in conjunction with or as a substitute for traditional data collection. The Professional Society for Health Economics and Outcomes Research [40]–International Society for Pharmacoepidemiology [41] (ISPOR-ISPE) Special Task Force has also provided recommendations to enhance clinical study practices and improve the acceptance of RWE by regulatory authorities, emphasizing good practices, transparency, and overall study conduct [42]. As expertise grows, researchers, clinicians, and regulators are likely to adopt and apply these designs on a larger scale [22]. Since the publication of the Operational, Technical, and Methodological (OPTIMAL) framework in 2019, there certain initiatives to incorporate selective usage of RWE for informing regulatory decisions by EMA.
Innovative approach to drug development planning
The development planning of rare disease trials necessitates a unique approach. Most of the companies still adhere to traditional clinical pharmacology approaches, which may not be as effective for rare diseases due to their unique characteristics and the small patient populations involved [43, 44]. As a result, many companies are adopting the oncological approach, where the product is tested in Phase I, particularly in patients with high morbidity or mortality. This approach allows the enrolment of a substantial number of patients and enables early assessment of the product’s safety and efficacy. This further helps in the reduction of overall trial costs and helps in resource optimization [45]. The data findings facilitate Model Informed Drug Development (MIDD), to enable addressing gaps in pharmacological studies and provide sufficient clinical data to initiate later phases of trials. This helps in better clinical evaluation and facilitates dose adjustments as well as endpoint modifications across different patient subpopulations. This approach also ensures that high-risk patients are not subjected to exclusion based on randomization and receive the treatment as part of the standard of care procedures [44, 45].
Small to medium-sized enterprises (SMEs) are integral to the development and distribution of treatments for rare diseases. These companies play a crucial role in optimizing logistics and leveraging expertise [46]. They often enter into commercial agreements with larger companies, facilitating initial developmental activities of the target therapy, performing an initial assessment of the product’s safety and efficacy, and transferring the same to larger entities for carrying out later activities. Through collaborative efforts, SMEs can access the resources and knowledge of larger companies, potentially accelerating the development and approval of treatments efficiently and effectively [47, 48]. This collaborative model can help to overcome the challenges associated with developing treatments for rare diseases, facilitating reduced timelines, and optimizing the financial and resource burdens, ultimately benefiting patients who may not have access to these life-saving therapies otherwise [47, 48].
Clinical trial research initiatives
Under the Seventh Framework Program (FP7), the European Union [2] funded three research projects to identify innovative trial designs for rare diseases. The challenges around small patient populations and heterogenous endpoints are recognized by researchers, industry, and regulators and these initiatives aimed to address these challenges and develop effective study methodologies and strategies to address these challenges. The studies aimed to improve the statistical methodology employed in trials involving a small number of patients through enhanced focus on the integration of trial design, conduct methodology, and outcomes and endpoint analysis. This approach ensures a comprehensive perspective and leads to refined methodologies that optimize the overall trial process [8]. The three projects have contributed to the implementation of study models and frameworks that are being increasingly adopted by researchers and sponsors to conduct rare disease clinical trials. Regulators have also shown increasing acceptance of novel methodologies and incorporating the findings in orphan drug approvals. More such initiatives focusing on specific therapeutic areas in coordination with ERNs will help in the development of other design methodologies that may address time criticality, can address endpoint adjustments, and modify the design based on continuous patient feedback. One key initiative that can be taken is a program similar to the Rare Disease Endpoint Advancement Program (RDEA) [49] by the US Food and Drug Administration (FDA). This is a dedicated program to advance the development of endpoints for clinical trials in rare diseases. This can be an EC-funded program under the supervision of EMA in collaboration with researchers, sponsors, and other regulatory authorities focusing on identifying and creating innovative, meaningful, and reliable endpoints to effectively assess rare disease treatment effectiveness. The EC-funded projects are listed below in Table 2:
Conclusion
The development of effective treatments for rare diseases remains a significant challenge due to the low prevalence and unique characteristics of these conditions. The challenges faced in clinical trials for orphan drugs in the EU are multifaceted and include patient recruitment, disease heterogeneity, limited knowledge of the natural history of rare diseases, lack of existing clinical study data, variability in disease characteristics, ethical considerations, cost challenges, and regulatory hurdles. Despite various financial incentives and grants extended to sponsors and researchers for rare disease research, all these challenges prove to be significant roadblocks in drug development as efficacy assessment becomes difficult due to the lack of test subjects and insufficient data that does not give enough evidence in favor of the medicine. Hence, close attention is required on an ongoing basis to address these issues. Due to the unique nature of rare diseases, there may not be a fit-for-all approach and traditional randomized trial methodologies prove ineffective in different settings. To mitigate these challenges, a few steps have been and can be taken, including the establishment of unified patient databases, PROMs, and RWE generation that has provided and has further potential to provide valuable information for drug development. Innovative approaches to clinical trial design, such as adaptive trial designs, basket and umbrella trials, and the use of real-world evidence, are being explored to address the challenges associated with rare disease trials. Apart from these, by embracing innovative approaches to drug development planning, utilizing external natural history study-based controls, using statistical modeling based on registry, standard of care, and natural history data, ensuring protocol flexibility, engaging patients, and incorporating RWD, researchers can overcome the unique challenges of rare disease trials and improve the development of effective therapies for patients with rare diseases. Continued collaboration between stakeholders, including researchers, clinicians, regulators, patient advocacy groups, and industry is required to foster continuous innovation and the proposed approaches aim to improve the efficiency, effectiveness, and generalizability of clinical research in the context of rare disease patient populations.
Data availability
All data used for analyzing the clinical trial landscape were accessed from the International Clinical Trials Registry Platform (ICTRP) of the World Health Organization. The data and related information are publicly available at: https://trialsearch.who.int/. All information generated during this study is presented and documented within the article itself. The data used and analyzed in the study is publicly available in Supplementary File 2. This includes all the necessary information and relevant data points used in the study.
Abbreviations
- CHMP:
-
Committee for Medicinal Products for Human Use
- COA:
-
Clinical Outcome Assessment
- DCT:
-
Decentralized Clinical Trial
- DHT:
-
Digital Health Technology
- EC:
-
European Commission
- EJP-RD:
-
European Joint Program on Rare Diseases (EJP-RD)
- EMA:
-
European Medicines Agency
- ERDRI:
-
European Rare Disease Registry Infrastructure
- ERICA:
-
European Rare Disease Research Coordination and Support Action Consortium
- ERN:
-
European Reference Network
- EU:
-
European Union
- EUCOPE:
-
European Confederation of Pharmaceutical Entrepreneurs
- EURORDIS:
-
European Organization for Rare Diseases
- ICH:
-
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use
- IRDiRC:
-
International Rare Diseases Research Consortium
- ISPOR-ISPE:
-
Professional Society for Health Economics and Outcomes Research–International Society for Pharmacoepidemiology
- NIH:
-
National Institute of Health
- OPTIMAL:
-
Operational, Technical, and Methodological Framework
- PROM:
-
Patient Reported Outcome Measures
- RCT:
-
Randomized Controlled Trial
- RDEA:
-
Rare Disease Endpoint Advancement Program
- RWD:
-
Real World Data
- RWE:
-
Real World Evidence
- RWE-RE:
-
Real World Evidence– Randomized Enrichment
- SAWP:
-
Scientific Advice Working Party
- SCA:
-
Synthetic Control Arm
- SOC:
-
Standard of Care
- US FDA:
-
US Food and Drug Administration
References
Committee for Medicinal Products for Human Use (CHMP). Guideline on clinical trials in small population [Internet]. European Medicines Agency. United Kingdom: European Medicines Agency; 2006 [cited 2023 May 24]. https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-trials-small-populations_en.pdf.
Talko M, Caudet L, European Commission. 2016 [cited 2023 Feb 16]. 7th Framework Programme for Research. https://ec.europa.eu/commission/presscorner/detail/en/MEMO_16_146.
Hilgers R. Design and analysis of clinical trials for small rare disease populations. J Rare Dis Res Treat. 2016;1(3):53–60.
Murphy MF, Applied Clinical T. 2016 [cited 2023 Apr 13]. Rare Diseases: Meeting the Unique Challenges of Orphan Drug Development. https://www.appliedclinicaltrialsonline.com/view/rare-diseases-meeting-unique-challenges-orphan-drug-development.
Motte S. de la. Synteract HCR. 2018 [cited 2023 Apr 20]. Orphan Indications and Clinical Trials - Why Rare Diseases Warrant Special Treatment. https://www.clinicalleader.com/doc/orphan-indications-and-clinical-trials-why-rare-diseases-warrant-special-treatment-0001.
ProRelix R. ProRelix Research. 2022 [cited 2023 Mar 31]. Planning a Rare Diseases Clinical Trial? View from United States and Europe. https://prorelixresearch.com/rare-disease-clinical-trial-in-us-europe-prorelix-research/.
Kempf L, Goldsmith JC, Temple R. Challenges of developing and conducting clinical trials in rare disorders. Am J Med Genet A. 2018;176(4):773–83.
O’Connor DJ, Hemmings RJ. Coping with small populations of patients in clinical trials. Expert Opin Orphan Drugs [Internet]. 2014;2(8):765–8. http://www.tandfonline.com/doi/full/https://doi.org/10.1517/21678707.2014.931221.
Bell SA, Tudur Smith C. A comparison of interventional clinical trials in rare versus non-rare diseases: an analysis of ClinicalTrials.gov. Orphanet J Rare Dis. 2014;9(1):170.
Ndebele P, Blanchard-Horan C, Shahkolahi A, Sanne I. Regulatory challenges Associated with conducting Multicountry clinical trials in resource-limited settings. JAIDS J Acquir Immune Defic Syndr. 2014;65(Supplement 1):S29–31.
McCormack P, Woods S, Aartsma-Rus A, Hagger L, Herczegfalvi A, Heslop E et al. Guidance in Social and Ethical Issues Related to Clinical, Diagnostic Care and Novel Therapies for Hereditary Neuromuscular Rare Diseases: Translating the Translational. PLoS Curr [Internet]. 2013; https://currents.plos.org/md/index.html%3Fp=2561.html.
ICTRP Secretariat. World Health Organization. 2018 [cited 2023 May 14]. International Clinical Trials Registry Platform (ICTRP). https://trialsearch.who.int/.
EJP RD INSERM. EJP-RD. [cited 2023 Mar 6]. European Joint Programme on Rare Diseases. https://www.ejprarediseases.org/.
EUCOPE. EUCOPE. 2017 [cited 2023 Mar 9]. European Confederation of Pharmaceutical Entrepreneurs (EUCOPE). https://www.eucope.org/.
The Scientific Secretariat of IRDiRC [Internet]. 2022 [cited 2023 Feb 12]. International Rare Diseases Research Consortium. https://irdirc.org/.
MAPI RESEARCH TRUST, MAPI RESEARCH TRUST. 2002 [cited 2023 Feb 20]. PROQOLID. https://eprovide.mapi-trust.org/about/about-proqolid#title-0.
ERICA Administrator. European Rare Disease Research Coordination and Support Action consortium (ERICA). 2022 [cited 2023 May 28]. WP3 Patient-Centred Research, ERICA. https://erica-rd.eu/work-packages/patient-centred-research/.
EURORDIS. EURORDIS. 2022 [cited 2023 May 23]. EURORDIS-Rare Diseases Europe. Proposal on the Revision of Orphan Medicinal Products and Paediatric Regulation. https://www.eurordis.org/publications/eurordis-proposal-on-the-revision-of-the-orphan-medicinal-products-and-paediatric-regulation/.
Day S, Jonker AH, Lau LPL, Hilgers RD, Irony I, Larsson K, et al. Recommendations for the design of small population clinical trials. Orphanet J Rare Dis. 2018;13(1):195.
Mellerio JE. The challenges of clinical trials in rare diseases. Br J Dermatol. 2022;187(4):453–4.
Rare Daily Staff. Global Genes. 2021 [cited 2024 Feb 5]. Bluebird Withdraws Gene Therapies in Europe as it Winds Down Operations There. https://globalgenes.org/raredaily/bluebird-withdraws-gene-therapies-in-europe-as-it-winds-down-operations-there/.
Baumfeld Andre E, Reynolds R, Caubel P, Azoulay L, Dreyer NA. Trial designs using real-world data: the changing landscape of the regulatory approval process. Pharmacoepidemiol Drug Saf. 2020;29(10):1201–12.
Mulberg AE, Bucci-Rechtweg C, Giuliano J, Jacoby D, Johnson FK, Liu Q, et al. Regulatory strategies for rare diseases under current global regulatory statutes: a discussion with stakeholders. Orphanet J Rare Dis. 2019;14(1):36.
Dreghici RD, Morris C, Servais L, Clinical L. 2023 [cited 2023 Jun 1]. New Advancement Toward The First Primary Endpoint Qualification Of A Digital Endpoint For Duchenne. https://www.clinicalleader.com/doc/new-advancement-toward-the-first-primary-endpoint-qualification-of-a-digital-endpoint-for-duchenne-0001.
Manolis E, Pons G. Proposals for model-based paediatric medicinal development within the current European Union regulatory framework. Br J Clin Pharmacol. 2009;68(4):493–501.
Cox GF. The art and science of choosing efficacy endpoints for rare disease clinical trials. Am J Med Genet A. 2018;176(4):759–72.
Crow RA, Hart KA, McDermott MP, Tawil R, Martens WB, Herr BE, et al. A checklist for clinical trials in rare disease: obstacles and anticipatory actions—lessons learned from the FOR-DMD trial. Trials. 2018;19(1):291.
Kakkis ED, O’Donovan M, Cox G, Hayes M, Goodsaid F, Tandon P, et al. Recommendations for the development of rare disease drugs using the accelerated approval pathway and for qualifying biomarkers as primary endpoints. Orphanet J Rare Dis. 2015;10(1):16.
Potgieter D, Kilian L, Bradham W, Merative US LP. 2023 [cited 2023 Aug 9]. Adaptive Clinical Trial Success Via A Unified Data Management And Acquisition Platform. https://www.merative.com/content/dam/merative/documents/white-paper/adaptive-clinical-trial-success-via-a-unified-data-management-and-acquisition-platform.pdf.
Maca J, Bhattacharya S, Dragalin V, Gallo P, Krams M. Adaptive seamless phase II/III Designs—Background, operational aspects, and examples. Drug Inf J. 2006;40(4):463–73.
Hilgers RD, Bogdan M, Burman CF, Dette H, Karlsson M, König F, et al. Lessons learned from IDeAl — 33 recommendations from the IDeAl-net about design and analysis of small population clinical trials. Orphanet J Rare Dis. 2018;13(1):77.
Eisenbeisz E, Omega S. 2018 [cited 2023 Jun 6]. Adaptive Seamless Design For Phase 2/3 Studies: Basic Concepts & Considerations. https://www.clinicalleader.com/doc/adaptive-seamless-design-for-phase-studies-basic-concepts-considerations-0001.
Moore J, Goodson N, Wicks P, Reites J. What role can decentralized trial designs play to improve rare disease studies? Orphanet J Rare Dis. 2022;17(1):240.
Haenel EK. 2022 [cited 2023 May 26]. Key Considerations For The Implementation Of Successful Decentralized Clinical Trials. https://www.clinicalleader.com/doc/key-considerations-for-the-implementation-of-successful-decentralized-clinical-trials-0001.
Ekangaki A, Premier R. 2023 [cited 2023 May 26]. Can Your Protocol Flex? The Importance Of Adaptive Trial Designs In Precision Oncology Studies. https://www.clinicalleader.com/doc/can-your-protocol-flex-the-importance-of-adaptive-trial-designs-in-precision-oncology-studies-0001.
Biostatistics WP, Modelling and, Party SW, Party PW. Scientific Advice Working Party. Use of extrapolation in the development of medicines for paediatrics [Internet]. European Medicines Agency. London: European Medicines Agency; 2018 [cited 2023 Jun 6]. https://www.ema.europa.eu/en/documents/scientific-guideline/adopted-reflection-paper-use-extrapolation-development-medicines-paediatrics-revision-1_en.pdf.
O’Connor D, Ciox Life S. 2021 [cited 2023 May 26]. Real-World Data Enhanced Clinical Trial Design. https://www.clinicalleader.com/doc/real-world-data-enhanced-clinical-trial-design-0001.
Loo EF. 2023 [cited 2023 May 26]. Speed Up, Collect More, And Reach Further: Using RWD To Optimize Your Clinical Trials. https://www.clinicalleader.com/doc/speed-up-collect-more-and-reach-further-using-rwd-to-optimize-your-clinical-trials-0001.
Myles J, Kamau A. Decentralized Trial Research Alliance (DTRA),Ikaika Health LLC. 2022 [cited 2023 May 26]. Using RWE In Clinical Trials: Challenges & Opportunities. https://www.clinicalleader.com/doc/using-rwe-in-clinical-trials-challenges-opportunities-0001.
ISPOR. The Professional Society for Health Economics and Outcomes Research. 2018 [cited 2023 May 18]. The Professional Society for Health Economics and Outcomes Research. https://www.ispor.org/heor-resources/about-heor.
INTERNATIONAL SOCIETY FOR PHARMACOEPIDEMIOLOGY [Internet]. [cited 2023 Jun 1]. International Society for Pharmacoepidemiology. https://www.pharmacoepi.org/.
Burns L, Roux N, Le, Kalesnik-Orszulak R, Christian J, Hukkelhoven M, Rockhold F, et al. Real-world evidence for Regulatory Decision-Making: Guidance from around the World. Clin Ther. 2022;44(3):420–37.
Ghadessi M, Di J, Wang C, Toyoizumi K, Shao N, Mei C, et al. Decentralized clinical trials and rare diseases: a Drug Information Association Innovative Design Scientific Working Group (DIA-IDSWG) perspective. Orphanet J Rare Dis. 2023;18(1):79.
Mitra A, Lee JB, Steinbach D, Hazra A, Krishna R. Rare oncology therapeutics: review of clinical pharmacology package of drug approvals (2019–2023) by US FDA, best practices and recommendations. J Pharmacokinet Pharmacodyn [Internet]. 2023 Dec 4 [cited 2024 Feb 9];50(6):475–93. https://link.springer.com/article/https://doi.org/10.1007/s10928-023-09896-2.
Iasonos A, O’Quigley J. Randomised phase 1 clinical trials in oncology. Br J Cancer. 2021;125(7):920–6.
Rollet P, Lemoine A, Dunoyer M. Sustainable rare diseases business and drug access: no time for misconceptions. Orphanet J Rare Dis. 2013;8(1):109.
Ascher J, M’lika A, Graf J, Prabhakaran M, McKinsey. & Company. 2021 [cited 2024 Feb 9]. Successful launches in rare diseases. https://www.mckinsey.com/~/media/McKinsey/Industries/Pharmaceuticals%20and%20Medical%20Products/Our%20Insights/How%20to%20successfully%20launch%20a%20rare%20disease%20drug%20in%20a%20patient%20centric%20world/Successful-launches-in-rare-diseases-vFinal.pdf
Alfano S, Amberg C, Peters N, Salazar P, Vieux-Rochas M, Welton S, McKinsey. & Company. 2023 [cited 2024 Feb 9]. Treating rare diseases: How digital technologies can drive innovation. https://www.mckinsey.com/industries/life-sciences/our-insights/treating-rare-diseases-how-digital-technologies-can-drive-innovation
Center for Drug Evaluation and Research. U.S. Food and Drug Administration. 2022 [cited 2022 Oct 29]. Rare Disease Endpoint Advancement Pilot Program. https://www.fda.gov/drugs/development-resources/rare-disease-endpoint-advancement-pilot-program.
Cordis, European Commission. 2017 [cited 2023 Jun 6]. Integrated Design and Analysis of clinical trials in SPG (IDeAl). https://cordis.europa.eu/project/id/602552/reporting.
Cordis, European Commission. 2017 [cited 2023 Jun 6]. Innovative methodology for small populations research. https://cordis.europa.eu/project/id/602144.
Baran-Kooiker A, Czech M, Kooiker C, OVERVIEW OF REGULATORY, INITIATIVES IN THE EUROPEAN UNION TO STIMULATE RESEARCH AND ACCELERATE ACCESS TO ORPHAN DRUGS AND OTHER HIGH MEDICAL NEED PRODUCTS. Acta Pol Pharm - Drug Res. 2019;76(1):3–17.
Cordis, European Commission. 2017 [cited 2023 Jun 6]. Advances in Small Trials Design for Regulatory Innovation and Excellence. https://cordis.europa.eu/project/id/603160.
Acknowledgements
Not applicable.
Funding
No Funding was received for this work.
Author information
Authors and Affiliations
Contributions
MP was responsible for conceptualizing the study and content. SM was responsible for data collection, analysis, and drafting of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mishra, S., Venkatesh, M. Rare disease clinical trials in the European Union: navigating regulatory and clinical challenges. Orphanet J Rare Dis 19, 285 (2024). https://doi.org/10.1186/s13023-024-03146-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-024-03146-5