- Research
- Open access
- Published:
The impact of FDA and EMA regulatory decision-making process on the access to CFTR modulators for the treatment of cystic fibrosis
Orphanet Journal of Rare Diseases volume 17, Article number: 188 (2022)
Abstract
Background
Over the past decade, a new class of drugs called CFTR (cystic fibrosis transmembrane conductance regulator) modulators have shown to be able to improve clinical outcomes in patient with Cystic Fibrosis. In this analysis, we have extensively reviewed the regulatory pathways and decisions adopted by FDA and EMA to speed up the development, the review and the approval of these drugs, with the aim of identifying possible clinical and public health implications associated with differences.
Results
CFTR modulators have been developed towards addressing three main genetic domains: (1) F508del homozygous (F508del/F508del), (2) F508del heterozygous, and (3) genotypes not carrying F508del mutation; and expanded from adult to paediatric population. Programs to expedite the reviewing and licensing of CFTR modulators were extensively adopted by FDA and EMA. All CFTR modulators have been licensed in the US as orphan drugs, but in the EU the orphan status for LUM/IVA was not confirmed at the time of marketing authorization as results from the pivotal trial were not considered clinically significant. While FDA and EMA approved CFTR modulators on the basis of results from phase III double-blind RCTs, main differences were found on the extension of indications: FDA accepted non-clinical evidence considering a recovery of the CFTR function ≥ 10% based on chloride transport, a reliable indicator to correlate with improvement in clinical outcomes. By contrast, EMA did not deem preclinical data sufficient to expand the label of CFTR modulators without confirmatory clinical data.
Conclusions
Regulators played an important role in fostering the development and approval of CFTR modulators. However, differences were found between FDA and EMA in the way of reviewing and licensing CFTR modulators, which extended beyond semantics affecting patients’ eligibility and access: FDA’s approach was more mechanistic/biology-driven while the EMA’s one was more oriented by clinical evidence. This might refer to the connection between the EMA and the Member States, which tends to base decisions on pricing and reimbursement on clinical data rather than pre-clinical ones. Here we have proposed a two-step personalized-based model to merge the ethical commitment of ensuring larger access to all potential eligible patients (including those harboring very rare mutations) with the one of ensuring access to clinically assessed and effective medicines through Real World Data.
Background
Cystic fibrosis (CF) is an autosomal recessive disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene affecting the functional expression of the CFTR protein, an ion channel that regulates the transport of chloride and bicarbonate at the cell surface [1]. Since the discovery of the CFTR gene in 1989 [2], more than 2000 mutations have been described, with different prevalence and severity of phenotype. Conventionally, mutations have been grouped into six classes: mutations introducing premature termination codons (i.e. frameshift, splicing, or nonsense mutations) (class I); misfolding mutations (class II); mutations hindering the regulation of the CFTR channel, also known as gating mutations (class III). These classes are usually associated with greater phenotypic severity and worse prognosis. On the other hand, mutations that entail milder clinical symptoms and better prognosis: mutations altering channel conductance (Class IV); mutations reducing the efficiency of CFTR production by affecting splicing (Class V), and mutations reducing the stability of mature CFTR at the cell membrane (Class VI) [3,4,5].
Early diagnosis and advances in symptomatic therapeutics aimed at dealing with major clinical complications—such as chronic airway infections and pancreatic insufficiency due to an abnormally thick and sticky mucus—have substantially improved the life-expectancy of CF patients [6].
However, better understanding of molecular and cellular pathology of CF paved the way for the development of a new class of drugs—called CFTR modulators—targeting the CFTR function directly [5]. As new agents were developed targeting different genotypes, limitations in the traditional class I–VI CF mutations system became evident and a new more drug-driven approach was developed and adopted by regulators for labelling therapeutic indications [1, 7, 8]. According to the US and the EU orphan legislations, CF is a rare disease and therefore drugs developed for its treatment are potentially eligible for Orphan Drug Designation (ODD), a special status that provides regulatory and financial incentives to sponsors to encourage drug development in unmet medical needs and non-profitable areas. Moreover, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established similar programmes to expedite the review and approval of medicines to treat serious and unmet medical need conditions such as CF. The aim of this study is to compare FDA and EMA approaches in the evaluation and approval of CFTR modulators and to identify possible clinical and public health implications associated with differences.
Methods
Data source and analysis
Qualitative and quantitative data regarding regulatory decisions (i.e. orphan drug designations, expedited programs, and approvals) by FDA and EMA on CFTR modulator medicinal products were retrieved from publicly accessible documents of the Register of FDA Approved Medicines [9] and from the register of medicinal products for human use authorized by the EU under the centralised procedure [10]. Data on evidence supporting regulatory decisions were further searched on the full prescribing information of FDA, the European public assessment report (EPAR) of EMA, clinicaltrials.gov, and published articles. Proofs were categorized into clinical and in vitro studies. Data from clinical studies included: phase and design of the study, eligible population (age and genotype), sample size, primary endpoint(s), outcome(s), and duration of the study/follow-up. Additionally, information on epidemiology and in vitro responsiveness of mutations to CFTR modulators were retrieved from CFTR2 database [11]—a website that provides information for patients, researchers, and the general public about specific variants in what is commonly referred to as the CF gene—and from the FDA and the EMA website, sponsor protocols, and scientific literature as appropriate. All data were updated as of January 30, 2022. Comparative analyses on orphan designations, and expedited programs granted by FDA and EMA were carried out, as well as those on decisions regarding eligible population (age and genotype), timing of decisions, and evidence (clinical studies vs in vitro studies) supporting approvals and variations of indications.
Classification of the CFTR mutations
Based on the pattern adopted by regulators, mutations have been categorized as follows: F508del, either as homozygous or heterozygous genotype; Gating, as already provided by Class III definition; Conduction, as already provided by Class IV definition; ‘Residual Function (RF)’; Minimal Function (MF); Other, which includes all the mutations not belonging to the above-mentioned categories (see Additional file 1). Acknowledgement of RF and MF mutations hinges upon predicted residual/minimal function of CFTR protein in keeping with population-level phenotypic data and in vitro response to CFTR modulators (IVA and TEZ). MF mutations show a severe phenotype—in fact, they lead to the complete absence of CFTR protein production or function—and do not respond in vitro to either IVA or TEZ, or TEZ/IVA [12, 13]; by contrast, RF mutations have a mild phenotype and respond to the above-mentioned CFTR modulators [14, 15]. Clinical severity has been defined as average sweat chloride (Sweat test—ST) ≥ 86 mmol/L, and prevalence of pancreatic insufficiency (PI) ≥ 50% [13], while in vitro response to CFTR modulators as an increase in percent normal chloride transport of ≥ 10 percentage points to transfected Fischer Rat Thyroid (FRT) cells expressing the CFTR form produced by the mutation [16]. In this study in vitro response data was obtained from assays performed on various cell models, i.e. FRT, CF Bronchial Epithelial (CFBE) and Human Nasal Epithelial (HNE) cell lines.
Published data on the in vitro response to CFTR modulators were searched on Pubmed as follows: < name of the mutation > AND < CFTR modulators > or < ivacaftor OR ivacaftor tezacaftor > . When clinical phenotype and in vitro response to CFTR modulators could not be matched to classify the mutation, it was categorized as Other. Two researchers performed the analysis and conflicts were solved through discussion with a third reviewer.
Results
Four products—developed and marketed by the same company—have been licensed in both the US and the EU: ivacaftor—IVA (Kalydeco®), a potentiator of CFTR function that increases the opening probability of the CFTR channel; lumacaftor/ivacaftor—LUM/IVA (Orkambi®), tezacaftor/ivacaftor—TEZ/IVA (Symdeko® in the US, Symkevi® in the EU), and elexacaftor/tezacaftor/ivacaftor—ELX/TEZ/IVA (Trikafta® in the US, Kaftrio® in the EU), as fixed combinations of potentiators and correctors that address the trafficking through the CFTR protein. All products were first licensed in the US: IVA 5.8 months before the EU approval; LUM/IVA 4.7 months; TEZ/IVA 8.7 months; ELX/TEZ/IVA 10.2 months. In respect of their different pathways, aims and year of launch, expedited programs were extensively adopted by FDA and EMA to foster the reviewing and licensing of CFTR modulators. While all CFTR modulators have been licensed in the US as orphan drugs, in the EU, the EMA’s Committee for Orphan Medicinal Products (COMP) did not recognize the results from the pivotal studies of LUM/IVA (despite statistically significant) as clinically relevant and did not confirm the orphan status at the time of marketing authorization (MA). LUM/IVA therefore missed the 10-year market exclusivity benefit [17]. In the US, expedited programs were implemented long before the development of CFTR modulators, while in the EU IVA and LUM/IVA were developed before PRIority MEdicines (PRIME) scheme came into force.
Anyway, neither in the US nor in the EU, CFTR modulators were considered eligible for earlier approval, as they were not granted accelerated approval by FDA and conditional marketing authorization by EMA respectively (see Additional file 2).
Expansion of indications
CFTR modulators have been developed towards addressing three main genetic domains: (1) F508del homozygous (F508del/F508del), (2) F508del heterozygous, and (3) genotypes not carrying F508del mutation. In keeping with their own functions and level of responsiveness to CFTR modulators, non-F508del mutations have been clustered in subgroups such as Gating, MF, RF and Other. A few conduction mutations have been described in medical literature, but the only one explicitly reported in approved therapeutic indications was R117H, as it exhibits a gating defect that was partially corrected by IVA.
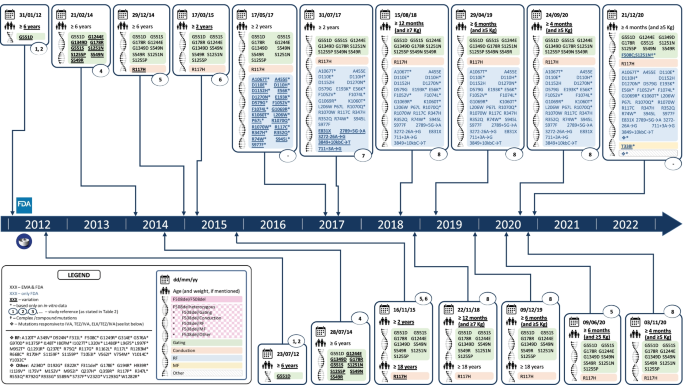
Over the past 10 years, therapeutic indications have expanded from a limited set to a wider array of mutations, and from individuals aged ≥ 12 years to paediatric population (Figs. 1 and 2). Whenever based on clinical data, FDA and EMA decisions were the same, except for the time delay and the eligible age for IVA of patients carrying the R117H mutation (FDA ≥ 12 years; EMA ≥ 18 years) at the time of licensing [18, 19] and those ≥ 12 years carrying F508del/RF genotype, in which an absolute mean change of + 4.7 (+ 3.7, + 5.8) from baseline in ppFEV1 at average of week 4 and 8 led to the approval in the US but not in the EU (VX14-661-108).
Chronogram of the marketing authorization and the extension of indications of LUM/IVA (a), TEZ/IVA (b) and ELX/TEZ/IVA (c) in the US and in the EU. Extensions of common indications FDA-EMA have been granted first by the FDA: LUM/IVA, 2 extensions in common, median 10.5 months (5.4–15.6); TEZ/IVA, 1 extension in common, 17.4 months before
Differences, on the other hand, have occurred in those cases where FDA adopted non-clinical evidence for its decision-making process (Table 1). To date, 183 out of 2106 (8.7%) described CF mutations [20] are explicitly eligible for CFTR modulators: 97 for IVA as monotherapy, 154 in combination with TEZ/IVA and 178 with ELX/TEZ/IVA; only 1 for LUM/IVA (F508del, as homozygote).
Clinical evidence
CFTR modulators have been licensed on the basis of results from phase III double-blind RCTs, whereas extensions of indications relied either on RCTs or on open-label single-group studies. Two studies regarding ELX/TEZ/IVA in patients ≥ 12 years F508del/F508del (VX17-445-03) and ≥ 12 years F508del/RF or F508del/Gating (VX18-445-04), provided an active comparator. Three studies—all regarding extensions of indication for IVA in patients 2–5 years and < 24 months patients harbouring Gating mutations, and LUM/IVA in patients 2–5 years F508del/F508del—were open-label single group CTs. The main primary endpoint adopted has been the absolute mean change from baseline in ppFEV1; however, in populations aged 6–11 years the lung clearance index (LCI2.5) was acknowledged as a more a sensitive measure of ventilation inhomogeneity, since it is able to detect early peripheral airway damage in CF patients with a greater sensitivity than spirometry. The two Agencies made consistent decisions on refusals, which regarded a phase II RCT (VX08-770-104) aimed at extending the indications of IVA to patients ≥ 12 years F508del/F508del and a phase III RCT (VX14-661-107) for the extension of TEZ/IVA to patients ≥ 12 years old F508del/MF, with an absolute mean change respectively of + 1.7 from baseline in ppFEV1 through week 16, and + 1.2 through week 12 (Table 2).
Non-clinical evidence
FDA has accepted in vitro studies for extending indications. Non-clinical endpoints taken into account include: a) the total ionic current (IT) due to the cell surface channel density; b) gating activity and conductance applied to FRT cells harbouring the mutation under study; c) the open channel probability (PO), representing the time interval when a single CFTR protein channel is open and transports ions; d) CFTR maturation, which relies on Western blotting techniques and monitors the cellular trafficking of CFTR to the apical surface. A recovery of the CFTR function ≥ 10% based on chloride transport has been considered reliable to lead to milder clinical manifestations of CF, i.e. a lower incidence of pancreatic insufficiency, and a more moderate lung function decline and lower sweat chloride levels, compared to patients with minimal CFTR chloride transport [21]. In 2017, after a previous rejection in 2016 [22], based on data from in vitro cell-based assays and on results from a previously exploratory phase IIa study, FDA granted 23 RF mutations as eligible for IVA [23, 24]. On the contrary, 26 MF mutations—not meeting the chloride transport threshold—were not approved [25]. As a post-marketing commitment, the Sponsor was required to conduct a 3-year single arm, observational study to further understand the clinical response to IVA in different subgroups of CF patients with CFTR mutations deemed responsive to IVA based on in vitro evidence [26]. Moreover, upon the same type of assays, mutation T338I—which had previously been refused—was approved for IVA (Fig. 1), TEZ/IVA and ELX/TEZ/IVA (Fig. 2) [27]. Overall, 82 mutations have been approved for IVA on the basis of in vitro assays. Following on the granting of RF mutations for IVA, in vitro assays were applied to other CFTR modulators.
TEZ/IVA was first licensed in the US on the basis of clinical evidence from in vitro studies. FRT assays were conducted on genotypes carrying IVA-responsive mutations (already approved in 2017) and F508del. TEZ/IVA showed a similar—rather than an increased—chloride transport level in comparison with IVA. However, any correlations of clinical benefit over IVA remained unclear. Subsequently, one of the two pivotal CTs—the crossover 3-treatment EXPAND study—confirmed the correlation of in vitro response to clinical efficacy of TEZ/IVA for 16 RF mutations. After the extension to patients ≥ 6 years, FDA approved an additional set of mutations through this pathway: in 2020, 127 additional mutations were granted eligibility for TEZ/IVA—quite surprisingly, 6 of these mutations (MF) were approved despite not being responsive in vitro—and 177 for ELX/TEZ/IVA (Fig. 2).
Discussion
CFTR modulators have been a groundbreaking and unprecedent achievement for CF. These small-molecules were first discovered through high-throughput screening (HTS) with medicinal chemistry interventions driven by predictive in vitro assays, and then brought into CTs. All CFTR modulators were first launched on the US market, which is the most remunerative pharmaceutical market in the world [28]. However, this might also refer to the fact that the sponsor is a US-based company and the initial development of CFTR modulators was supported by the US CF Foundation (CFF), thus, exerting some sort of pressure on the company's marketing strategy and policy [29]. Differences were also found in regulatory approaches, partly due to the legal background of the two systems, partly related to scientific principles, culture of weighing benefits and risks, and dealing with the subsequent Health Technology Assessment (HTA) evaluations [30]. For example, COMP’s decision on confirmation of the ODD for LUM/IVA at the time of MA steered Member States’ assessment on value-based pricing and reimbursement: HTA organizations emphasized that a < 4% change in FEV1 was not a relevant clinical outcome, since its correlation to pulmonary exacerbations (PEX) or to other clinically relevant outcomes remains unclear [31].
Development strategy for CFTR modulators
The clinical development of CFTR modulators expanded from the monotherapy of the potentiator IVA in a selected group of Gating mutations[32, 33], to the last combination ELX/TEZ/IVA [21] targeting the CFTR function in patients carrying at least one F508del mutation [34, 35]. The overarching approach adopted for studying CFTR modulators in clinical stage was short-term double-blind RCTs followed by open label long-term safety studies [36, 37]. Meanwhile, investigations moved towards the paediatric population, providing extrapolated data from older population as well as assessing pharmacokinetics, safety and tolerability [38, 39]. Efficacy has been found heterogeneous across different CFTR modulators and targeted population, but—remarkably—FDA and EMA adopted the same decisions for both approvals or refusals.
Results from IVA targeting G551D mutation [36] were considered the benchmark for the development of following CFTR modulators: accordingly, LUM/IVA and TEZ/IVA showed a modest clinical benefit, while ELX/TEZ/IVA was recognized as the standard of care for genotypes carrying at least one F508del mutation, namely the most frequent allelic variant in CF: with slight geographical differences, current CFTR modulators could target on average 70–80% of CF patients [40, 41]. By way of contrast, 20–30% of patients are not yet eligible for treatments, and this percentage includes: a) rare mutations neither enrolled in CTs nor studied, and therefore not licensed by regulators; b) mutations whose biological features prevent the use of CFTR modulators, i.e. premature stop mutations producing truncated unstable mRNA and a lack of full-length CFTR proteins, so that the use of CFTR modulators would be not plausible.
Predictable models
The extrapolation of data from preclinical models to expand the clinical use of CFTR modulators has been the major difference found between FDA and EMA [42]. As ion transport properties of primary human CF respiratory epithelial cells can be preserved in cell cultures, non-clinical studies have been used as proof-of-concept to demonstrate the preliminary efficacy of CFTR modulators [43].
Testing modulators on a variety of laboratory or patient-derived cells (Theratyping) has the potential of characterizing complex CFTR variants, of assessing modulator responsiveness of rare/unique CFTR mutations, and even of providing an optimization in the modulator therapy regimen through modulator responsiveness comparison. Patient-derived model systems may avoid the challenges of varying responses to CFTR modulators within the same genotype among different patients (for the purpose of a personalized therapy) and can support the selection of suitable “likely responders to drug” subgroups to be enrolled in CTs through the characterization of unclassified CFTR variants by the response to modulators [44]. For example, a strong correlation between in vitro data and clinical outcomes has been observed with IVA in patients carrying F508del mutation: < 10% recovery of CFTR was subsequently confirmed as a non-statistically significant increase for FEV1. Although stringent criteria must be met before considering the use of in vitro data alone to expand a drug indication—such as a good understanding of the disease and a solid comprehension of the drug’s mechanism of action [45]—not all situations have confirmed such correlation: LUM/IVA showed a very promising 25.1% recovery of CFTR functionality in F508del genotypes but a modest increment in FEV1 [46]. These discrepancies have raised doubts on the validity of preclinical models and their use for regulatory approvals. In the EU, preclinical data were accepted for granting initial ODD, a stage where non-clinical studies were considered reliable for anticipating clinical effects of new products. By contrast, at the time of MA only confirmed clinical data were acknowledged.
Implications for patients and healthcare systems
Differences between FDA and EMA in the way of reviewing and licensing medicines lay on procedures and relevant clinical decisions. In oncology, for example, the Agency that provided a positive opinion was found to be more restrictive in terms of wording indications compared with the Agency that first granted approval [47]. In the case of CF, differences extend beyond semantics, procedures and timing of approval, but affect patients’ eligibility. In the US, extensions of indication based on in vitro data have addressed patients carrying mutations not included in CTs because of their rarity [48]. Since then, new and alternative predictable models have been implemented. Recent advances in adult stem cell biology have produced the development of organoids using a variety of tissue sources such as intestine, respiratory epithelium and kidney [48]. When no approved treatments are available for rare mutations, and large scale CTs are therefore not feasible, n-of-1 trials have been proposed to contribute to the totality of evidence for expanding drug indications. However, this approach has some methodological strengths and weaknesses to be carefully considered before supporting expansion of access to expensive medicines [49].
A stepwise model to merge drug-regulation and HTA
As regulators have to manage the challenge of uncertainty in the benefit/harm assessment, systems of personalized therapy might progressively support regulatory decisions and subsequent HTA evaluations. An increased coordination between these two levels may promote a new and more flexible model that could fall under the tag of ‘payment at results’ agreements. The introduction of Next-Generation Sequencing in clinical practice has opened new perspective for precision and personalized medicines. In oncology, the need for systematic interpretation of molecular alterations and their translation into clinical practice has been addressed thorough the implementation of the so-called ‘Molecular Tumour Boards’: a panel of experts who analyse tumour genotypes in order to recommend the most suitable targeted therapy [50, 51]. A ‘CF Molecular Board’ could be implemented, with a view to promoting an efficient and timely manner access to CFTR modulators to patient carrying mutations not included in CTs. Genotypes might be screened and when considered eligible for CFTR modulators, they should be candidate to theratyping tests, the results of which should be confirmed in clinical setting. Efficient use is essential for public health funded systems, in particular in the case of orphan drugs which are tagged at high nominal price [52]. This approach can meet both the ethical imperative of taking care of individual patients and the recognition of a value-based price.
Reviewing pharmaceutical R&D funding for rare diseases
Despite the unquestionable advances in the treatment of CF and the potential impact of personalized approaches to target further rare mutations, a significant number of individuals in the world do not benefit from CFTR modulators. And there is more to come. New CFTR modulators and new innovative approaches are currently in development to target patients who have experienced limited benefits from already approved CFTR modulators and also for targeting non-sense mutations [53].
Orphan legislations and incentive systems have brought a huge contribution to target unmet medical needs, also in CF. But it is time now to rethink and set sustainable policies for the future, by ensuring R&D programs to meet patients’ needs, as well as equitable access to the innovations. In the US, new approaches have been recently implemented on public–private collaboration to foster the delivery of new gene therapies to patients affected by ultra-rare diseases [54].
However, also in the EU, one of the four pillars of the ‘Pharmaceutical strategy for Europe’ aims at ensuring access to affordable medicines for patients, and at addressing unmet medical needs, such as CF [55].
In the area of (ultra)rare diseases, experimenting with public–private partnerships throughout the life-cycle of a drug could better address its development towards medical needs, so mitigating and sharing business risks and dealing with failures, and most importantly steering the pricing of drugs once they are placed on the market, in order to improve their affordability and subsequent access for patients.
Public and global health outlook
The epidemiological profile of CF has been changing. Advances in the management of the disease have increasingly transformed what was considered an exclusive pediatric disease into an adult disorder. On the other hand, epidemiological studies have shown that CF extend beyond the US and the EU boundaries. Given its higher prevalence among Caucasians, CF has long been considered an exclusive disease of western countries. However, data on CFTR mutations have been progressively reported from Asia, the Middle East, Latin America and Africa [56]. The incidence of CF in Low-Income Countries (LICs) is variable and depends on the composition and origin of the population, and the awareness of the condition which inevitably leads to its underdiagnosis, misdiagnosis, and underreporting. However, nowadays effective medicines are available and their patents are expiring, which can globally lead to an improvement of their affordability. Meanwhile, confirmatory results from clinical use of CFTR modulators on rare mutations might also contribute to maximizing the cost–benefit profile of these medicines in LICs. But in Western Countries several challenges remain, especially for HTA where comparative CTs and the contribution of Real-World Evidence (RWE) are expected to increasingly contribute to better define the place in therapy of different treatments and their value (and hence their accessibility).
Conclusions
Our analysis has brought valuable insights on the regulatory decision-making process of FDA and EMA on CFTR modulators for the treatment of CF, emphasizing the role of regulators in fostering the development and approval of these medicines and the streamlined access to a growing number of patients. Remarkably, FDA took the unusual decision of expanding the use of CFTR modulators on the basis of data from in vitro. By contrast, EMA did not deem preclinical data sufficient to expand the label of CFTR modulators without clinical data.
Such differences raise an important question: what should drive the approval of new drugs or a new indication? Clinical evidence or biological markers? We proposed a two-step personalized-based model to merge the ethical commitment of ensuring larger access to all potential eligible patients (as provided by FDA) with the one of ensuring access to clinically assessed and effective medicines (as provided by EMA).
As most of the novel medicines that have been introduced in clinical practice globally are first approved by FDA and EMA, the two-step approach we have proposed here—to confirm biological plausibility in clinical practice within a reimbursement agreement—can provide a more comprehensive amount of knowledge for an incremental cost-effective use of CFTR modulators worldwide.
Availability of data and materials
Please contact author for data requests.
References
Shteinberg M, Haq IJ, Polineni D, Davies JC. Cystic fibrosis. Lancet. 2021;397:2195–211.
Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989;245:1073–80.
Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science. 1992;256:774–9.
Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001.
Lopes-Pacheco M. CFTR modulators: the changing face of cystic fibrosis in the era of precision medicine. Front Pharmacol. 2019;10:1662.
Cohen-Cymberknoh M, Shoseyov D, Kerem E. Managing cystic fibrosis: strategies that increase life expectancy and improve quality of life. Am J Respir Crit Care Med. 2011;183:1463–71.
de Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4:662–74.
Marson FAL, Bertuzzo CS, Ribeiro JD. Classification of CFTR mutation classes. Lancet Respir Med. 2016;4:e37–8.
U.S. Food and Drug Administration (FDA). Drugs@FDA: FDA-Approved Drugs. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm. Accessed 30 Jan 2022.
EU Union Register of medicinal products for human use. https://ec.europa.eu/health/documents/community-register/html/reg_hum_act.htm?sort=a. Accessed 30 Jan 2022.
The Clinical and Functional TRanslation of CFTR (CFTR2). https://cftr2.org/. Accessed 30 Apr 2021.
Munck A, Kerem E, Ellemunter H, Campbell D, Wang LT, Ahluwalia N, et al. Tezacaftor/ivacaftor in people with cystic fibrosis heterozygous for minimal function CFTR mutations. J Cyst Fibros. 2020;19:962–8.
Vertex Pharmaceuticals Incorporated. VX17-445-102, Version 3.0 [Study protocol]. https://clinicaltrials.gov/ProvidedDocs/44/NCT03525444/Prot_000.pdf. Accessed 30 Jan 2022.
Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, et al. Tezacaftor–Ivacaftor in residual-function heterozygotes with cystic fibrosis. N Engl J Med. 2017;377:2024–35.
Vertex Pharmaceuticals Incorporated. VX18-445-104, Version 2.0 [Study protocol]. https://clinicaltrials.gov/ProvidedDocs/53/NCT04058353/Prot_000.pdf. Accessed 30 Jan 2022.
Vertex Pharmaceuticals Incorporated. VX14-661-108, Version 3.0, Appendix A [Study protocol]. https://clinicaltrials.gov/ProvidedDocs/34/NCT02392234/Prot_000.pdf. Accessed 30 Jan 2022.
UE Committee for Orphan Medicinal Products (COMP). EMA/COMP/46160/2016 Rev.2: Minutes for the meeting on 6–8 October 2015. https://www.ema.europa.eu/en/documents/minutes/minutes-comp-meeting-6-8-october-2015_en.pdf.
Vertex Pharmaceuticals Incorporated. Kalydeco (ivacaftor) [Full Prescribing Informations]. Revised December 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/203188s014s015lbl.pdf. Accessed 30 Apr 2021.
EU Committee for Medicinal Products for Human Use (CHMP). EMA/670896/2020 Corr.1—Kalydeco (ivacaftor) [EPAR—Assessment Report—Variation]. https://www.ema.europa.eu/en/documents/variation-report/kalydeco-h-c-2494-ii-0027-epar-assessment-report-variation_en.pdf.
Cystic Fibrosis Mutation Database (CFTR1). http://www.genet.sickkids.on.ca/cftr/StatisticsPage.html. Accessed 30 Jan 2022.
UE Committee for Medicinal Products for Human Use (CHMP). EMA/385871/2020 Rev.1—Kaftrio (ivacaftor/tezacaftor/elexacaftor) [EPAR—Assessment Report]. https://www.ema.europa.eu/en/documents/assessment-report/kaftrio-epar-public-assessment-report_en.pdf.
Kingwell K. FDA OKs first in vitro route to expanded approval. Nat Rev Drug Discov. 2017;16:591–2.
Durmowicz AG, Lim R, Rogers H, Rosebraugh CJ, Chowdhury BA. The U.S. Food and Drug Administration’s experience with ivacaftor in cystic fibrosis. Establishing efficacy using in vitro data in Lieu of a clinical trial. Ann Am Thorac Soc. 2018;15:1–2.
Vertex Receives Complete Response Letter from U.S. FDA for Use of KALYDECO® (ivacaftor) in People with Cystic Fibrosis Ages 2 and Older with One of 23 Residual Function Mutations. [Press release]. Business Wire. 2016. https://www.businesswire.com/news/home/20160205005336/en/. Accessed 30 Jan 2022.
Vertex Pharmaceuticals Incorporated. Kalydeco (ivacaftor) [Full Prescribing Informations]. Revised May 2017. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207925s001lbl.pdf. Accessed 30 Jan 2022.
US Center for Drug Evaluation and Research (CDER). Kalydeco (ivacaftor) [Supplemental approval]. 2017. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2017/203188Orig1s019,207925Orig1s001ltr.pdf. Accessed 30 Jan 2022.
Vertex Pharmaceuticals Incorporated. Kalydeco (ivacaftor) [Full Prescribing Informations]. Revised December 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/203188s034,207925s013lbl.pdf. Accessed 30 Jan 2022.
Global pharmaceutical sales from 2017 to 2020, by region. Statista. https://www.statista.com/statistics/272181/world-pharmaceutical-sales-by-region/. Accessed 25 Sept 2021.
Ramsey BW, Nepom GT, Lonial S. Academic, foundation, and industry collaboration in finding new therapies. N Engl J Med. 2017;376:1762–9.
Vreman RA, Naci H, Goettsch WG, Mantel-Teeuwisse AK, Schneeweiss SG, Leufkens HGM, et al. Decision making under uncertainty: comparing regulatory and health technology assessment reviews of medicines in the United States and Europe. Clin Pharmacol Ther. 2020;108:350–7.
Vreman RA, de Ruijter AS, Zawada A, Tafuri G, Stoyanova-Beninska V, O’Connor D, et al. Assessment of significant benefit for orphan medicinal products by European regulators may support subsequent relative effectiveness assessments by health technology assessment organizations. Drug Discov Today. 2020;25:1223–31.
de Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J Cyst Fibros. 2014;13:674–80.
Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an Arg117His-CFTR mutation: a double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–33.
Middleton PG, Mall MA, Dřevínek P, Lands LC, McKone EF, Polineni D, et al. Elexacaftor–tezacaftor–ivacaftor for cystic fibrosis with a single Phe508del Allele. N Engl J Med. 2019;381:1809–19.
Barry PJ, Mall MA, Álvarez A, Colombo C, de Winter-de Groot KM, Fajac I, et al. Triple therapy for cystic fibrosis Phe508del-Gating and -residual function genotypes. N Engl J Med. 2021;385:815–25.
Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72.
Bessonova L, Volkova N, Higgins M, Bengtsson L, Tian S, Simard C, et al. Data from the US and UK cystic fibrosis registries support disease modification by CFTR modulation with ivacaftor. Thorax. 2018;73:731–40.
Davies J, Sheridan H, Bell N, Cunningham S, Davis SD, Elborn JS, et al. Assessment of clinical response to ivacaftor with lung clearance index in cystic fibrosis patients with a G551D-CFTR mutation and preserved spirometry: a randomised controlled trial. Lancet Respir Med. 2013;1:630–8.
Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous for F508del-CFTR: a randomised, placebo-controlled phase 3 trial. Lancet Respir Med. 2017;5:557–67.
Orenti A, Zolin A, van Rens J, Fox A, Krasnyk M, Daneau G, et al. ECFS patient registry 2019. 2021. https://www.ecfs.eu/sites/default/files/general-content-images/working-groups/ecfs-patient-registry/ECFSPR_Report_2019_v1_23Dec2021.pdf.
Cystic Fibrosis Foundation (CFF). Cystic fibrosis foundation patient registry—2020 annual data report. https://www.cff.org/media/23476/download.
Amaral MD, de Boeck K. ECFS Strategic Planning Task Force on ‘Speeding up access to new drugs for CF’. Theranostics by testing CFTR modulators in patient-derived materials: the current status and a proposal for subjects with rare CFTR mutations. J Cyst Fibros. 2019;18:685–92.
van Goor F, Hadida S, Grootenhuis PDJ, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–30.
Crawford KJ, Downey DG. Theratyping in cystic fibrosis. Curr Opin Pulm Med. 2018;24:612–7.
U.S. Food and Drug Administration (FDA). Novel approach allows expansion of indication for cystic fibrosis drug [Press release]. 2017. https://www.fda.gov/drugs/news-events-human-drugs/novel-approach-allows-expansion-indication-cystic-fibrosis-drug. Accessed 30 Jan 2022.
Ponzano S, Nigrelli G, Fregonese L, Eichler I, Bertozzi F, Bandiera T, et al. A European regulatory perspective on cystic fibrosis: current treatments, trends in drug development and translational challenges for CFTR modulators. Eur Respir Rev. 2018;27:66.
Trotta F, Leufkens HGM, Schellens JHM, Laing R, Tafuri G. Evaluation of oncology drugs at the European Medicines Agency and US Food and Drug Administration: when differences have an impact on clinical practice. J Clin Oncol. 2011;29:2266–72.
Berkers G, van Mourik P, Vonk AM, Kruisselbrink E, Dekkers JF, de Winter-de Groot KM, et al. Rectal organoids enable personalized treatment of cystic fibrosis. Cell Rep. 2019;26:1701-1708.e3.
Magaret AS, Mayer-Hamblett N, VanDevanter D. Expanding access to CFTR modulators for rare mutations: the utility of n-of-1 trials. J Cyst Fibros. 2020;19:1–2.
Roychowdhury S, Iyer MK, Robinson DR, Lonigro RJ, Wu Y-M, Cao X, et al. Personalized oncology through integrative high-throughput sequencing: a pilot study. Sci Transl Med. 2011;3:111–21.
Mateo J, Chakravarty D, Dienstmann R, Jezdic S, Gonzalez-Perez A, Lopez-Bigas N, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895–902.
Luzzatto L, Hyry HI, Schieppati A, Costa E, Simoens S, Schaefer F, et al. Outrageous prices of orphan drugs: a call for collaboration. Lancet. 2018;392:791–4.
Cystic Fibrosis Foundation (CFF). Drug Development Pipeline. https://apps.cff.org/trials/pipeline/. Accessed 28 Dec 2021.
Kingwell K. “Bespoke Gene Therapy Consortium” sets out to enable gene therapies for ultra-rare diseases. Nat Rev Drug Discov. 2021;20:886–7.
Leufkens HG, Kusynová Z, Aitken M, Hoekman J, Stolk P, Klein K, et al. Four scenarios for the future of medicines and social policy in 2030. Drug Discov Today. 2022;6:66.
Bell SC, Mall MA, Gutierrez H, Macek M, Madge S, Davies JC, et al. The future of cystic fibrosis care: a global perspective. Lancet Respir Med. 2020;8:65–124.
Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187:1219–25.
Flume PA, Liou TG, Borowitz DS, Li H, Yen K, Ordoñez CL, et al. Ivacaftor in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Chest. 2012;142:718–24.
Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2–5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4:107–15.
Rosenfeld M, Wainwright CE, Higgins M, Wang LT, McKee C, Campbell D, et al. Ivacaftor treatment of cystic fibrosis in children aged 12 to <24 months and with a CFTR gating mutation (ARRIVAL): a phase 3 single-arm study. Lancet Respir Med. 2018;6:545–53.
Davies JC, Wainwright CE, Sawicki GS, Higgins MN, Campbell D, Harris C, et al. Ivacaftor in infants aged 4 to <12 months with cystic fibrosis and a gating mutation. Results of a two-part Phase 3 clinical trial. Am J Respir Crit Care Med. 2021;203:585–93.
Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor–ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–31.
McNamara JJ, McColley SA, Marigowda G, Liu F, Tian S, Owen CA, et al. Safety, pharmacokinetics, and pharmacodynamics of lumacaftor and ivacaftor combination therapy in children aged 2–5 years with cystic fibrosis homozygous for F508del-CFTR: an open-label phase 3 study. Lancet Respir Med. 2019;7:325–35.
Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor–Ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377:2013–23.
Davies JC, Sermet-Gaudelus I, Naehrlich L, Harris RS, Campbell D, Ahluwalia N, et al. A phase 3, double-blind, parallel-group study to evaluate the efficacy and safety of tezacaftor in combination with ivacaftor in participants 6 through 11 years of age with cystic fibrosis homozygous for F508del or heterozygous for the F508del-CFTR mutation. J Cyst Fibros. 2021;20:68–77.
Heijerman HGM, McKone EF, Downey DG, van Braeckel E, Rowe SM, Tullis E, et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: a double-blind, randomised, phase 3 trial. The Lancet. 2019;394:1940–8.
Zemanick ET, Taylor-Cousar JL, Davies J, Gibson RL, Mall MA, McKone EF, et al. A Phase 3 open-label study of Elexacaftor/Tezacaftor/Ivacaftor in children 6 through 11 years of age with cystic fibrosis and at least one F508del Allele. Am J Respir Crit Care Med. 2021;203:1522–32.
Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX-445-Tezacaftor–Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1612–20.
EU Committee for Medicinal Products for Human Use (CHMP). EMA/CHMP/567306/2018: Symkevi (tezacaftor/ivacaftor) [EPAR—Assessment Report]. https://www.ema.europa.eu/en/documents/assessment-report/symkevi-epar-public-assessment-report_en.pdf.
Acknowledgements
None.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
EC: conceived of the study, participated in its design, collected data, performed the analyses interpreted the data, and drafted the manuscript; SG: participated in the design, collected and interpreted the data and draft the manuscript; FP: participated in the design, interpreted the genetic data; HL: participated in the planning of analyses and interpretation of results; LL: conceived of the study, participated in the planning of analyses and interpretation of results; All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
EC: member of the Committee for Orphan Medicinal Products (COMP) at EMA. The views expressed in this article are the personal views of the author and may not be understood or quoted as being made on behalf of or reflecting the position of the regulatory agency with which the author is affiliated. SG: the author declares that she has no competing interests. FP: the author declares that she has no competing interests. HL: former chair of the Dutch Medicines Evaluation Board (MEB), former member of several committees and working parties of the European Medicines Agency (EMA). MC: received grants from Vertex Ph (ISS), from the Italian Minister of Health (COVID-2020-12371781) and from Pfizer (ID_61509709). He also served on advisory boards for Vertex Ph, Chiesi, Viatris, Kither.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Algorithm adopted to classify the eligible mutations to CFTR modulators as approved by FDA and EMA. Complete phenotypic data comprehends: average sweat chloride (sweat test, ST) (mmol/L) and pancreatic insufficiency in percentage (PI%). Criteria for severe phenotype are ST ≥ 86 mmol/L, and PI% ≥ 50%. Definitions: * = if mentioned; WT-CFTR = wild-type CFTR protein; RF = residual function CFTR mutation; MF = minimal function CFTR mutation. Other: a = Uncomplete/missing phenotypic data; b = conflicting phenotypic; c = conflicting phenotypic/responsiveness data.
Additional file 2.
Framework for fostering the development, review and approval of medicines for rare and serious life-threatening conditions in the US and in the EU. Definitions: MA = Marketing Authorization, SMEs = small & medium-sized enterprises. At the time of marketing authorization LUM/IVA was withdrawn from the Community Register of designated Orphan Medicinal Products of the EU upon request of the sponsor [25]. In the EU, the designation to accelerated assessment - which shortens the review time from 210 to 150 days - was granted to IVA and LUM/IVA, while the EMA did not agree to the applicant’s request for TEZ/IVA being considered not of major public health interest [69]. The triple combination ELX/TEZ/IVA was initially reviewed under EMA’s accelerated assessment program, but since the applicant requested a 3-month clock stop during assessment - ultimately reduced to 2 months - the conditions for accelerated assessment could no longer be met [35].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Costa, E., Girotti, S., Pauro, F. et al. The impact of FDA and EMA regulatory decision-making process on the access to CFTR modulators for the treatment of cystic fibrosis. Orphanet J Rare Dis 17, 188 (2022). https://doi.org/10.1186/s13023-022-02350-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13023-022-02350-5